USP <90> Compendial Quality & Function Testing of Fetal Bovine Serum

 *The materials required to perform the USP Radial Immunodiffusion have been discontinued and no current updates to the USP allow for a suitable replacement. We are actively evaluating validation of an alternative method per General Notices 6.30 of the USP. For more information, please contact your Business Development Rep/Project Manager, or contact us here.
*The materials required to perform the USP Radial Immunodiffusion have been discontinued and no current updates to the USP allow for a suitable replacement. We are actively evaluating validation of an alternative method per General Notices 6.30 of the USP. For more information, please contact your Business Development Rep/Project Manager, or contact us here.
Fetal Bovine Serum (FBS), as a byproduct of the cattle industry, is one of the preferred animal sera for cell culture proliferation. With an abundance of protein, growth factors, enzymes and other chemical components, and a characteristically low or absent concentration of interfering antibodies, FBS is ideal for promoting cell health and growth.
Eurofins BioPharma Product Testing network of laboratories has established a broad array of physical, chemical, electrophoretic, and immunochemical techniques for determination of quality attributes of sera. With substantial experience and expertise in cell culture needs, we can support serum functionality testing, including collaborative design and execution of user-defined testing. Eurofins BioPharma Product Testing's vast capacity and extensive capabilities can provide clients with rigorous quality attribute testing and critical functionality testing to ensure fast-growing healthy cell lines, making animal sera a practical quality controlled means for cell culture enhancement.
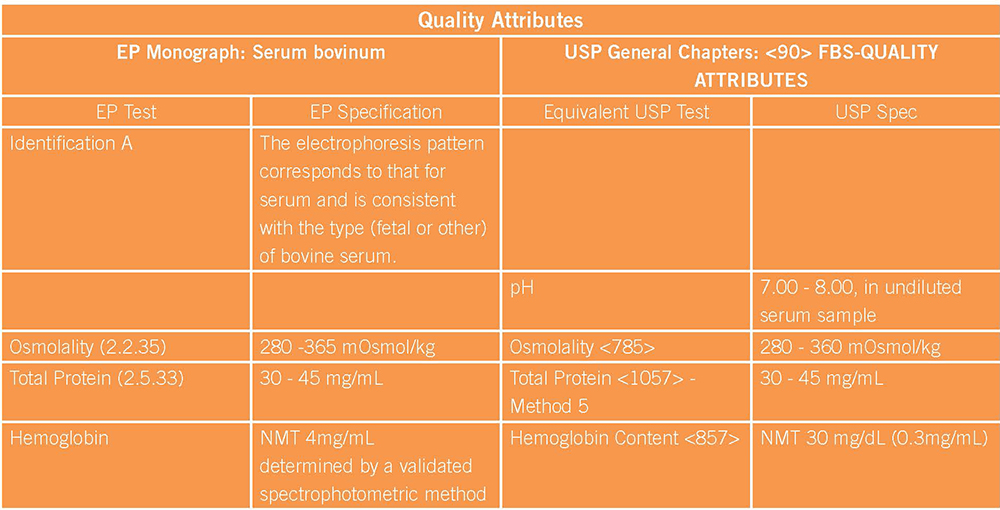
In an effort to provide guidance and standards for use, the European Pharmacopeia (EP) and US Pharmacopeia (USP) have released general Quality Attributes and Functionality Tests for FBS. The Quality Attributes Tests provide standards to ensure consistency in protein content, hemoglobin level, pH, osmolality, IgG, species identification, and electrophoretic profile. The Functionality Testing outlined by the compendia can be performed with specified cell lines, comparing cell growth in test sera supplemented media versus reference sera supplemented media. However, in many cases, clients will favor establishment of their own user-defined functionality assays to ensure that test sera supports appropriate growth of their own cell line.
Why Choose Eurofins BioPharma Product Testing?
- We qualify reagents in cell culture and cell banking activities before the use of the reagents to ensure high quality results, saving time and money for clients.
- With extensive expertise and capacity, we provide clients the full range of testing at one location, meeting all media components needs.
- We are the only laboratory offering full USP <90> testing with recommended cell lines, as well as custom functional testing.
Testing Capabilities/Methods
Eurofins BioPharma Product Testing provides a full range of testing to meet all media component needs, including:
- 9 CFR (bovine and porcine)
- In vitro screening
- Mycoplasma and Sterility Testing
- Chemistry
Contact Us
Request more information to start working with us today.
















































