E&L Programs for Complex Dosage Forms and High-Risk Drug Products

 Understanding the regulatory requirements for Extractable & Leachable (E&L) studies can lead to confusion, and if the inappropriate path is taken, it could result in delays in getting a drug into the market. Deficiencies most commonly arise when the expectations for material characterization or “simple” E&L studies are applied to the more complex drug delivery systems. Much of the confusion stems from the varying requirements and expectations based on a drug’s route of administration, formulation, dosing requirement, container/closure system, and drug delivery device requirements. This leaves many companies asking if they need to follow PQRI, ISO 10993, USP 661/662, USP 1663/1664, EMEA, BPOG, BPSA, or any other number of regulatory guidance publications. As a result, inadequate E&L information in filings has continued to be a significant source of deficiencies cited in Complete Response Letters such as1:
Understanding the regulatory requirements for Extractable & Leachable (E&L) studies can lead to confusion, and if the inappropriate path is taken, it could result in delays in getting a drug into the market. Deficiencies most commonly arise when the expectations for material characterization or “simple” E&L studies are applied to the more complex drug delivery systems. Much of the confusion stems from the varying requirements and expectations based on a drug’s route of administration, formulation, dosing requirement, container/closure system, and drug delivery device requirements. This leaves many companies asking if they need to follow PQRI, ISO 10993, USP 661/662, USP 1663/1664, EMEA, BPOG, BPSA, or any other number of regulatory guidance publications. As a result, inadequate E&L information in filings has continued to be a significant source of deficiencies cited in Complete Response Letters such as1:
- Presence of E&L compounds above the Qualification Threshold (QT) that have not been identified
- Inadequate sensitivity to be able to detect compounds at the requested Analytical Evaluation Threshold (AET)
- Inadequate stability data to examine trends in leachables over time
- Inadequate descriptions of how extractable data were used to design leachable assessments
- Inadequate E&L correlations
With over 20 years of experience performing E&L studies, Eurofins BioPharma Product Testing has developed and validated over 1,000 leachable methods and has experience with every route of administration and common container-closure system on the market. We have completed over 50 inhalation programs (pMDI, DPI, Nasal Sprays and nebulizers), and over 100 complex drug/device programs (Transdermal patches, implantables, auto-injectors, etc.).
Why Choose Eurofins BioPharma Product Testing?
- We provide full E&L program support, including controlled extraction studies, leachable method development/validation, extractable/leachable identification, toxicological evaluations, and leachable stability studies.
- We custom design E&L programs to meet clients’ specific needs and that also meet current regulatory standards and expectations.
- We use a proprietary HPLC-MS identification database, Eurofins Extractable Index, with over 1,500 compounds (including common plasticizers, anti-oxidants, stabilizers, elastomers, lubricants, and accelerants) to identify extractables and/or leachables in products.
- We provide regulatory consultative services to help our clients navigate the regulatory expectations and perform risk assessments of their manufacturing chains and/or container/closure systems.
- We have a successful regulatory track record with no rejections or delays in approval from a Eurofins designed E&L program.
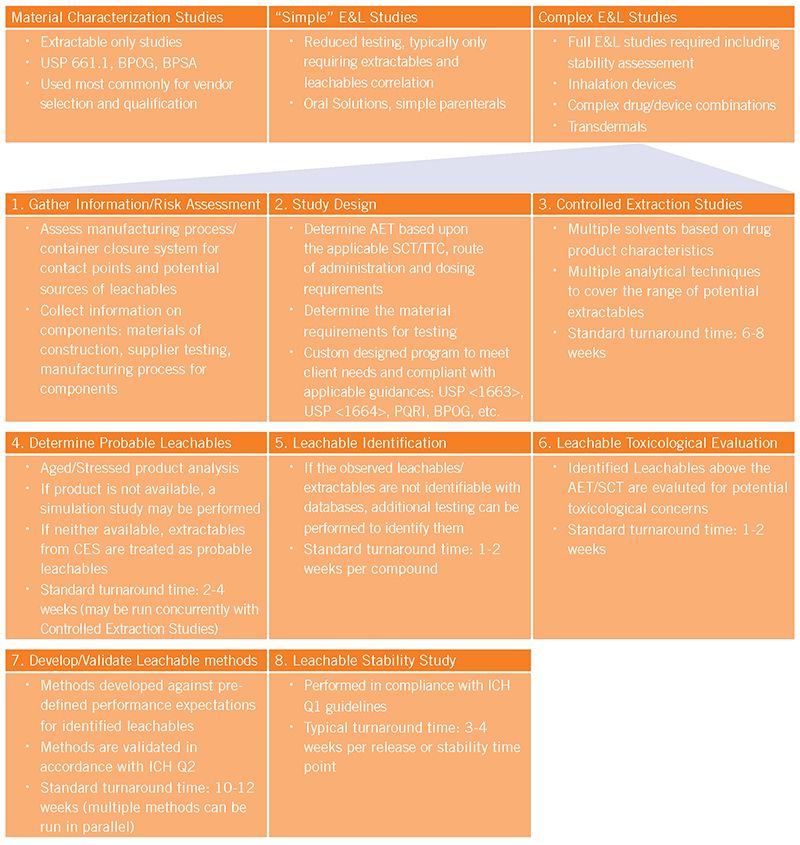
E&L studies can generally break down into three categories listed below. The flow chart helps to outline the basic steps required to successfully complete an E&L study for the most complex products.
1Mellon, Dan (May, 2019) Nonclinical Review of Extractable Leachable Studies: Practical Advice from an FDA Reviewer, Extractables & Leachables USA, Arlington, VA.
Contact Us
Request more information to start working with us today.
















































