Accredited dietary Ingredients GMP Process Certification to 21 CFR 111 / 117 and NSF/ANSI 173, Section 8 – 2024


Dietary ingredients form the fundamental part of the quality and safety of dietary supplements which are highly regulated by FDA when manufactured in or exporting to the U.S. market. Choosing Good Manufacturing Practices (GMPs) certification is a proactive approach that demonstrates your dietary ingredients’ compliance to regulations, establishing stronger trust among buyers and consumers.
At Eurofins Assurance, we offer dietary ingredients GMP certification to important US regulations for food and supplement manufacturing, including 21 CFR Part 111, 21 CFR 121, 21 CFR Part 117 (Subparts A, B, & F), and 21 CFR Part 1 (Subpart L) and NSF/ANSI 173 – 2024 Section 8. This set of requirements ensures best practices in manufacturing traceability and documentation and helps ingredient manufacturers meet the needs of their regulated dietary supplements manufacturer customers. This programme is accredited to ISO/IEC 17065 by ANAB for 21 CFR 111/117.
Certifications requirements for dietary ingredients 21 CFR 111 / 117 and NSF/ANSI 173, Section 8 – 2024
Section 201(ff) (1) of the FD&C Act (21 U.S.C 321(ff) (1)) states a dietary ingredient is a vitamin; a mineral; an herb or other botanical; an amino acid; a dietary substance for use by man to supplement the diet by increasing total dietary intake; or a concentrate, metabolite, constituent, extract, or combination of any of the above dietary ingredients.
There are no standards tailored for dietary ingredient manufacturing, which are regulated as food. Food regulations alone simply do not assure the level of traceability and documentation the dietary supplements industry depends on to mee their own strict regulatory requirements in the US market.
To fill this gap, Eurofins Assurance has developed a voluntary, custom protocol that is a blend of food and supplement regulations and best practices, to help ingredient manufacturers differentiate from the competition. Through this certification, ingredient manufacturers can show they have committed to implementing best practices and are prepared to support their customers regulatory compliance.
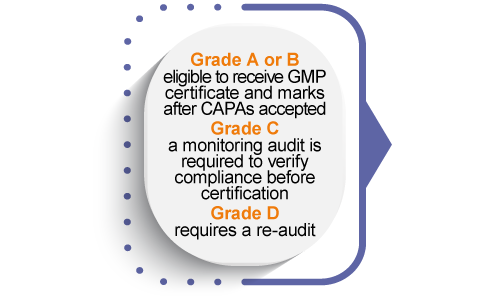
A GMP certificate of conformity and the Eurofins Assurance certification marks are issued to the company after all program requirements are met and the company is able to market its certification its customers and distinguish itself from competitors.
Dietary ingredients GMP certification process overview
To initiate the certification process, Eurofins will collect detailed information about your company manufacturing sites, including some pre audit documentation to ensure preparedness and proper audit scope and duration is agreed.
The audit duration is based upon the scope and complexity of the operation but is usually 2-3 days for a single location.







Eurofins Assurance Dietary Ingredients GMP Process Certification Documentation
Manufacturers who meet all certification requirements receive a GMP Certificate of Conformity issued by Eurofins Healthcare Assurance. Our GMP Certificates are accepted by some renowned retailers and brands as conformity document.
Companies are also licensed to use and display the Eurofins Dietary Ingredients GMP Certification Mark on corporate marketing materials to communicate the achievement and highlight the company commitment to quality and continuous improvement.
Please refer to the Policies and Procedures for Good Manufacturing Practices (GMPs) and Management Systems Certification. The certification is conducted by Eurofins Assurance Audit and Certification Services US, LLC and the Terms & Conditions can be viewed here.

Why choosing Eurofins for your dietary supplement GMP certification
With experts in the US and around the world, the Eurofins Healthcare Assurance team have decades of experience with dietary and food supplements regulations and manufacturing best practices to support your FDA compliance and meet retailer and brand expectations. Our team are third party conformity assessment experts and leverage the most experienced, well-trained auditors located globally to support customers with geographic proximity.
For dietary supplements GMP certification, Eurofins Assurance also offers:
- GRMA certification (NSF/ANSI 455-2 for Dietary Supplements)
- GMP process certification to 21 CFR 111/117 and NSF/ANSI 455-2-2024
- SSCI certification
Our certification activities are provided by independent Certification Bodies separately from any consulting activities. Impartiality is safeguarded by Eurofins Assurance’s relevant policies to avoid conflicts of interest.

This programme is accredited to ISO/IEC 17065 by ANAB for 21 CFR 111/117, demonstrating competence, impartiality, and credibility. You can view relevant scope of accreditation from the ANSI National Accreditation Board (ANAB) here.




© Eurofins Assurance 2025 Personal data protection policy

















































