Accredited cosmetic GMP process certification to ISO 22716 or NSF/ANSI 455-3


Looking for GRMA certification on NSF/ANSI 455-3 for Cosmetics? Visit here.
While cosmetics regulation vary globally, trends towards increasing regulation and certification requirements is widespread.
Cosmetics have traditionally enjoyed lighter regulation compared to other categories like food and drugs. However, increasingly complex supply chains, endless novelty in the cosmetic product space in forms and ingredients, and changes in regulation such as the Modernization of Cosmetics Regulation Act of 2022 (MoCRA) in the US, along with increased consumer awareness and perception of quality risks related to these products, has changed the risk profile associated with manufacturing, distributing, and offering these products for retail sale.
While the trend towards increased oversight continues, there remains differences in the most accepted standards depending on your market. To best meet the needs of our globally located clientele, Eurofins Assurance offers two cosmetic scopes under it GMP process certification program, ISO 22716 and NSF/ANSI 455-3, enabling a choice of that which bests meets your business needs. This programme is accredited to ISO/IEC 17065 by ANAB.
As both options fall under the Eurofins Healthcare GMP Certification program, each certification follows a single process. All that remains is to choose the best fit:
Certification requirements of ISO 22716:2007
ISO 22716:2007 is the most widely accepted standard globally for Cosmetic GMP. ISO 22716 GMPs in this standard cover personnel, premised, equipment, production from raw material to distribution, the laboratory, good documentation practices, and most internationally recognized components of a quality management system including complaints, recalls, change control, and internal audits.
Certification requirements of NSF/ANSI 455-3 2024
NSF/NSI 455-3 is normative to ISO 22176, and so certification to this standard also provides certification to ISO 22716. However, as the American National Standard for Cosmetics, this standard is designed to align with best practices expected in a US market, and so includes previous FDA Cosmetics GMP Guidance, and new MoCRA regulatory requirements as they are implemented. Manufacturers importing cosmetic product into the US, may consider choosing this certification to demonstrate preparedness for stringent US regulations and to meet US retailer expectations, or those manufacturers who seek to demonstrate conformity with best practices.
Compliance with ISO 22716 and NSF/ANSI 455-3 demonstrates a commitment to cosmetic product safety and adherence to international guidelines, improving quality management systems.
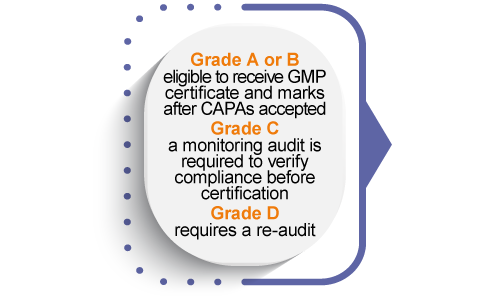
A GMP certificate of conformity and the Eurofins Assurance certification Marks are issued to the after all program requirements are met, after which the company can market its certification to its customers and distinguish itself from competitors.
GMP certification process overview
To initiate the certification process, Eurofins will collect detailed information about your company manufacturing sites, including some pre-audit documentation to ensure preparedness and proper audit scope and duration is agreed.
The audit duration is based upon the scope and complexity of the operation but is usually 2-3 days for a single location.







Eurofins Assurance Cosmetic GMP Process Certification Documentation
Manufacturers who meet all certification requirements receive a GMP Certificate of Conformity issued by Eurofins Healthcare Assurance.
Companies are also licensed to use and display the Eurofins Cosmetic ISO 22716 or NSF/ANSI 455-3 GMP Certification Mark on corporate marketing materials to communicate the achievement and highlight the company commitment to quality and continuous improvement.
Please refer to the Policies and Procedures for Good Manufacturing Practices (GMPs) and Management Systems Certification. The certification is conducted by Eurofins Assurance Audit and Certification Services US, LLC and the Terms & Conditions can be viewed here.


Why choosing Eurofins for your cosmetic GMP certification
With experts in the US and around the world, the Eurofins Assurance team possesses strong expertise in certification and GMPs. Our team are third party conformity assessment experts and leverage the most experienced, well-trained auditors located globally to support customers with geographic proximity.

This programme is accredited to ISO/IEC 17065 by ANAB, demonstrating competence, impartiality, and credibility. You can view relevant scope of accreditation from the ANSI National Accreditation Board (ANAB) here.
Our certification activities are provided by independent Certification Bodies separately from any consulting activities. Impartiality is safeguarded by Eurofins Assurance’s relevant policies to avoid conflicts of interest.




© Eurofins Assurance 2026 Personal data protection policy

















































