Biopharmaceutical Release Testing

 To support your commercial product and clinical trial material release testing needs, the Eurofins BioPharma Product Testing network of laboratories offer the capacity and breadth of capabilities to test your formulated bulk, final product or in-process materials in a timely manner.
To support your commercial product and clinical trial material release testing needs, the Eurofins BioPharma Product Testing network of laboratories offer the capacity and breadth of capabilities to test your formulated bulk, final product or in-process materials in a timely manner.
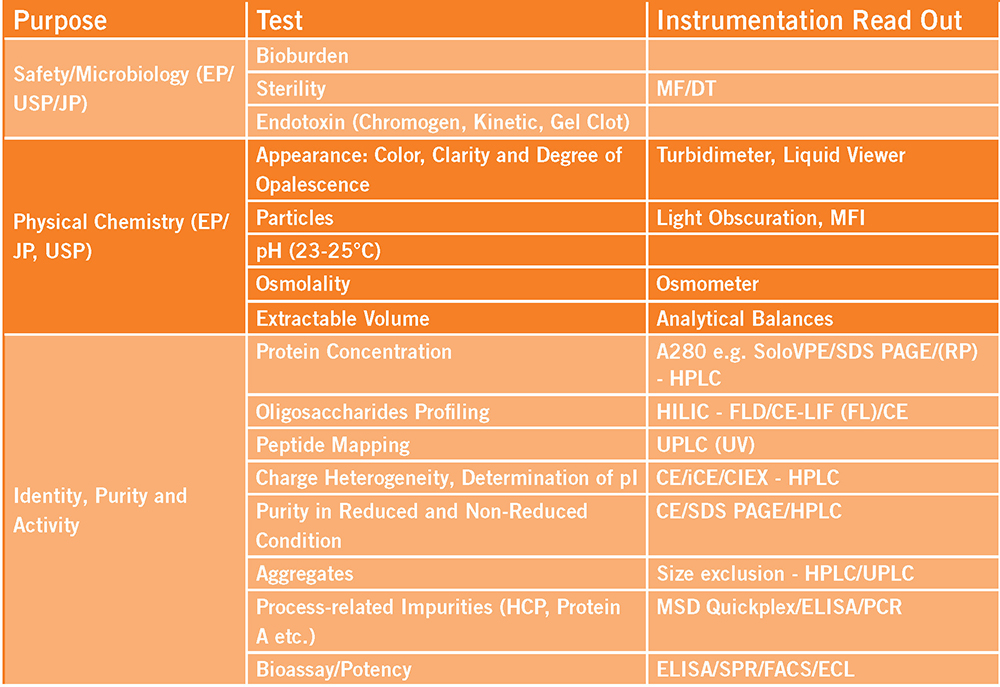
We test materials against specifications for identity, purity, potency, impurities, physical properties and safety under strict cGMP compliance, and we customize individual programs to streamline lab documentation and reporting for the most efficient and quality-focused data deliverable possible.
Our multi-shift laboratory operations are designed to provide extensive capacity for larger volume release programs, as well as flexibility for smaller programs in a manner that allows for aggressive cycle times.
Our release testing services are backed by an outstanding scientific approach to problem solving and extensive expertise in Method Development and Validation.
Why Choose the Eurofins BioPharma Product Testing?
- We have in-depth experience working with many modality types and have the flexibility and extensive instrument capacity within our team to meet the ever-changing demands of production schedules and timelines.
- We have multiple centers of excellence globally for development, optimization, and transfer of in vitro bioassays for potency.
- Our breadth of capabilities allows us to perform all testing of even the most complex conjugated molecules through one preferred partner.
- Our global capabilities allow us to support your EU batch release requirements.
Our Experience Includes
- Therapeutic Proteins (MAbs, Biosimilars, Fusion & Recombinant)
- Synthetic Peptides
- Therapeutic Enzymes
- Conjugates
- Cell/Gene Therapy
- Vaccines
Potency/Bioassay Services
- Development and validation of cell-based bioassays using multiple formats, including absorbance, fluorescence, time-resolved fluorescence, luminescence and electrochemical luminescence as well as binding and competitive ELISAs.
- Assay optimization to eliminate sources of variability and ensure consistent performance for QC release.
- Multiple software packages available for data analysis, including Softmax Pro and StatLIA.
Instrumentation
- HPLC/UPLC
- UV
- qPCR
- CE/iCE
- Plate Readers
- ECL
- Liquid handling system, including plate washer and reader
- MFI
- Mass Spectrometry
- KF
Release Testing Capabilities