Physico-Chemical Characterization of Biopharmaceutical Products

 Characterization testing is utilized to gain an understanding of the physical and chemical properties of biopharmaceutical materials. During process and drug development, these properties can have an impact on the product’s performance, ability to be processed, stability and appearance. Therefore, a well characterized biopharmaceutical is integral to moving a candidate through development and to the market. Due to the complex nature of these materials, extensive testing utilizing a wide array of techniques is required.
Characterization testing is utilized to gain an understanding of the physical and chemical properties of biopharmaceutical materials. During process and drug development, these properties can have an impact on the product’s performance, ability to be processed, stability and appearance. Therefore, a well characterized biopharmaceutical is integral to moving a candidate through development and to the market. Due to the complex nature of these materials, extensive testing utilizing a wide array of techniques is required.
Eurofins BioPharma Product Testing network of laboratories provides complete characterization packages according to regulatory guidelines for biopharmaceutical products, such as the ICHQ6B guideline, to support formulation or process development, comparability studies, reference material (re)qualifications, and investigational manufacturing troubleshooting.
Why Choose Eurofins BioPharma Product Testing?
- Our extensive experience with a variety of sample types lends the ability to tailor the testing to meet specific client requirements at one testing location
- Our laboratories are equipped with state-of-the-art instrumentation
- Our Quality System offers complete electronic data traceability and high compliance level
- Our teams have decades of experience supporting complete development/clinical program using release assays and supportive methods
Experience
- Therapeutic Proteins and Glycoproteins
- Monoclonal Antibodies
- Vaccines
- Antibody-Drug Conjugates (ADCs)
- Peptides and Conjugated Peptides
- Enzymes
- Fusion Proteins
- Oligonucleotides
- Virus-like Particle (VLP)
- Cell and Gene Therapy Products
Characterization Testing Capabilities
Physico-chemical properties
- Intact Molecular weight (ESI-MS, LC-MS, SEC-UV, SEC-MALS, MALDI-TOF MS)
- Intact proteins, subunits (reduced form), and domains (IdeS enzyme)
- Glycosylated and deglycosylated proteins
- Identification of isoforms and glycoforms
- ADCs Drug to Antibody Ratio (DAR) calculation
- Proteins and pegylated proteins
- Amino acid analysis for concentration determination and Extinction Coefficient Estimation
- Protein concentration (Amino Acid Analysis, SoloVPE, A280)
- Thermal unfolding (Nano-DSC)
Primary structures and Post-Translational Modifications
- Peptide mapping including:
- Confirmation of amino acid sequence
- Sequence coverage
- Post translational Modifications evaluation
- Disulfide bond mapping
- Free Thiol Analysis (Ellman’s)
- Glycosylation analysis
- Monosaccharide quantitative analysis
- Sialic acid quantitative analysis
- N- and O-glycan chromatographic profiling
- N- and O-glycan mass spectrometry structural elucidation
High Order Structures: Secondary and Tertiary
- Circular Dichroism (Near and far UV)
- FTIR (ATR and Transmission)
- Intrinsic and Extrinsic Fluorescence
Aggregation
- SEC-UV, SEC-RI, SEC-MALS
- SV-AUC
- DLS (incl. Zeta Potential evaluation)
Particle analysis
- DLS (incl. Zeta Potential evaluation)
- MFI
Product-related impurity evaluation
- Charges variants by HPLC techniques
- Size variants (CE-SDS, CE-LIF, SDS-PAGE, Native-PAGE, SEC-HPLC, SEC-MALS)
- Hydrophobic profiles by RP and HIC chromatography
Product-related impurity identification
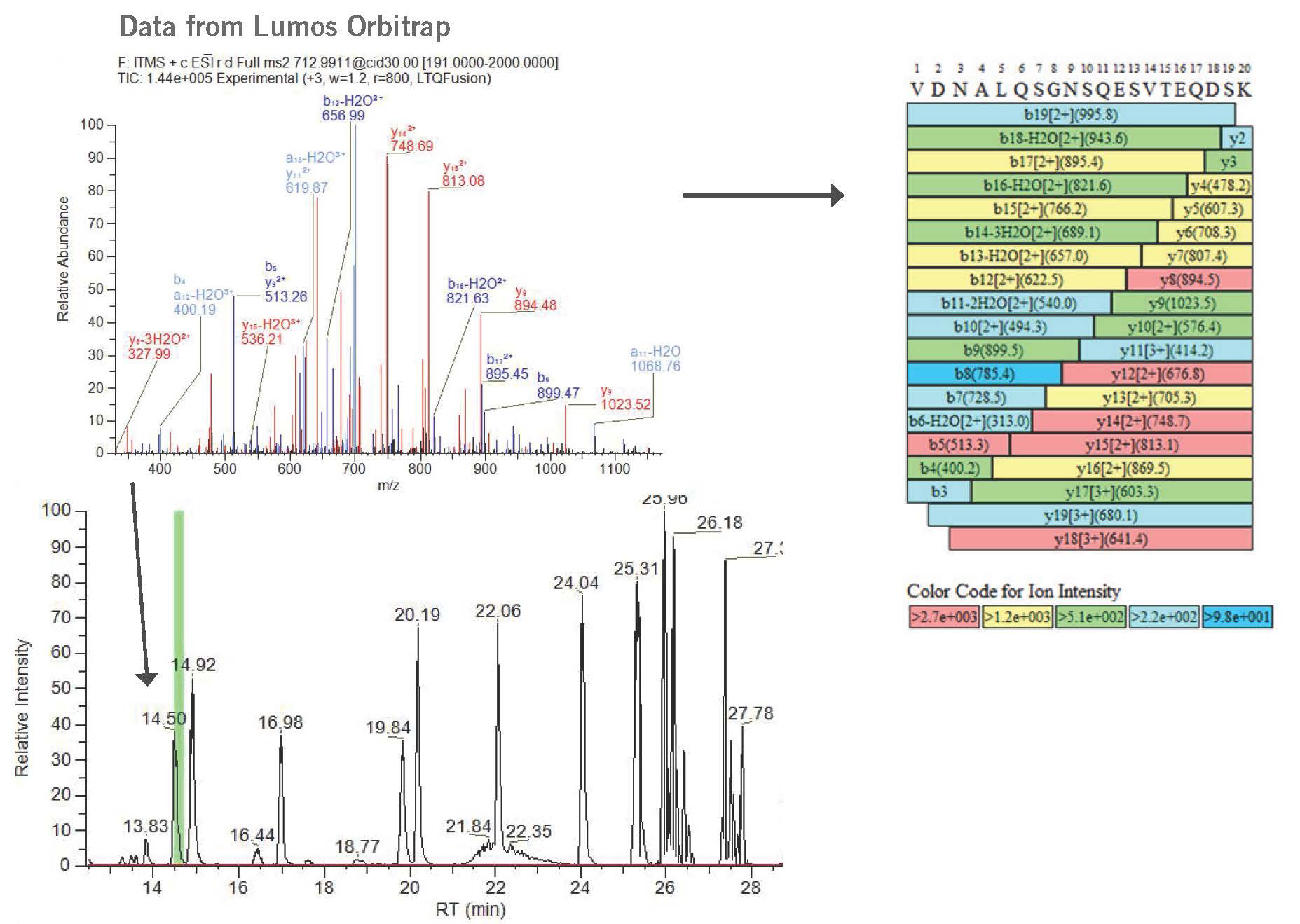
- Fraction collection and identification of unknown peaks using peptide mapping strategy and NanoLC-MS/MS (High Resolution Mass spectrometry instrument such as Thermo Lumos Orbitrap)
Product-related impurity evaluation
- Residual testing
- Residual HCP identification and selective quantitation by Mass Spectrometry
- Gel-band identification by Proteomics techniques and databases interrogation